How Does CFC Works?
Published at - November 17, 2021
7 min read
How Does CFC Works?
The full form of CFC is Chlorofluorocarbon. It isn’t a deadly gas. It doesn’t kill us, but that only makes it stronger. CFC is and has always been a natural enemy of the ozone layer. The Ozone layer, by absorbing damaging ultraviolet-B (UV-B) rays from the sun, blankets the entire planet and safeguards life on Earth. Like any saviour, it has a lot of enemies, and often, ironically, many of these enemies are manufactured by the very soul it protects. We, by our ruthless act and pointless tyranny, manufacture the enemies of the Ozone layer like CFC.
Preventing any disasters require knowledge and insight. To save the ozone layer that protects us, we must know about its enemies, how it is produced and how it is annihilated. In this blog, that is exactly what we are gonna try to do. We will discuss what CFC is, how it is built and what havoc it causes. Moreover, we shall try to find out the ways to abolish this enemy or reduce it to a tolerable level.
What is CFC?
CFCs and HCFCs are fully or partially halogenated paraffin hydrocarbons that contain solely carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), and are created as volatile derivatives of methane, ethane, and propane. They're also known by the brand name Freon, which is a DuPont product. Dichlorodifluoromethane is the most typical example (R-12 or Freon-12). Many CFCs have been employed as refrigerants, propellants (for aerosols), and solvents.
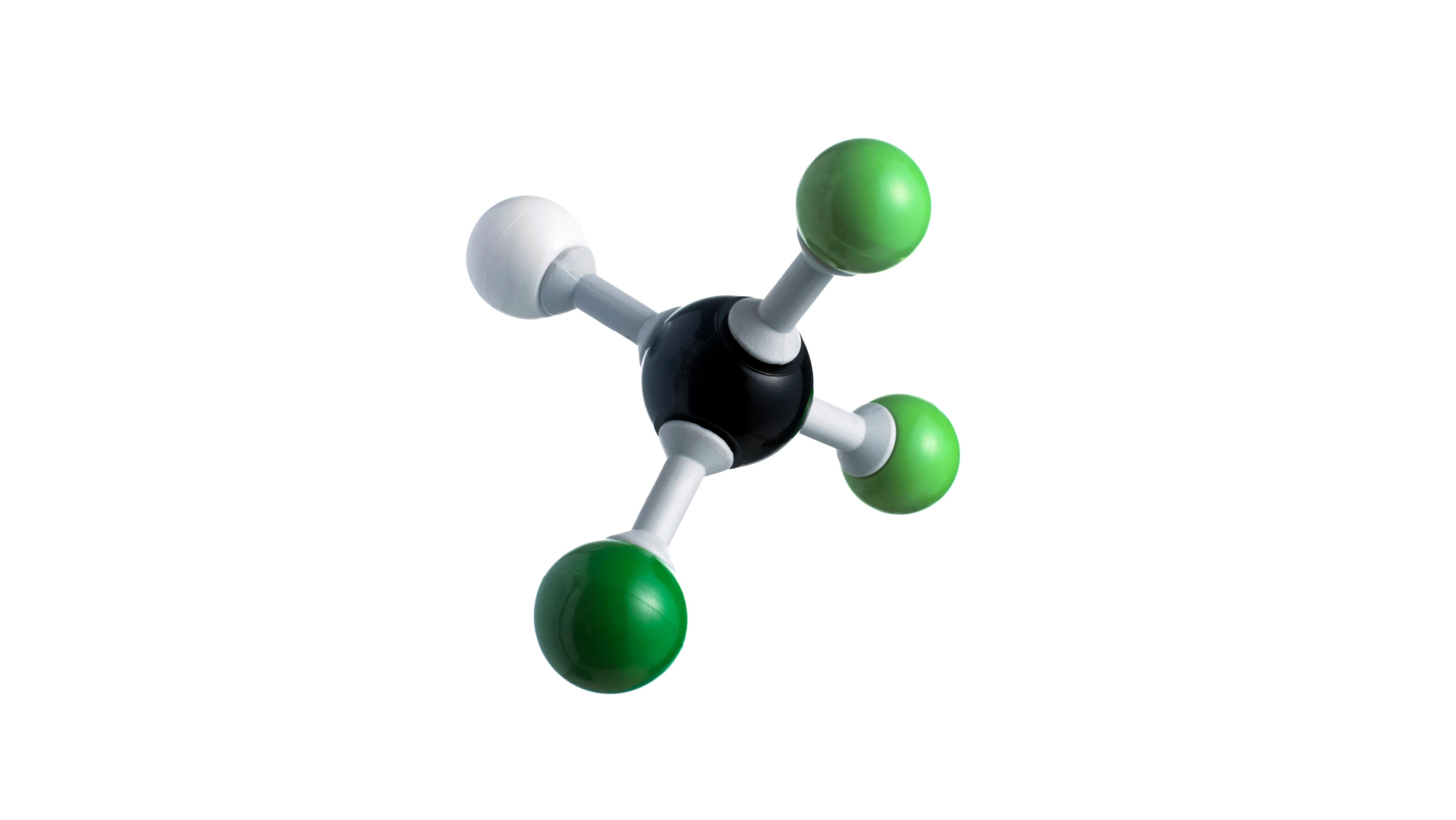
Carbon in CFCs bonds with tetrahedral symmetry, just like in simpler alkanes. The methane-derived CFCs deviate from ideal tetrahedral symmetry because the fluorine and chlorine atoms differ substantially in size and effective charge from hydrogen and each other. Changes in the amount and identity of the halogen atoms can alter the physical properties of CFCs and HCFCs. They are volatile in general, but not as much as their parent alkanes. The lower volatility is due to the halides' induction of molecular polarity, which causes intermolecular interactions. Methane boils at 161 degrees Celsius, while fluoromethanes boil between 51.7 (CF2H2) and 128 degrees Celsius (CF4). Because chloride is more polarizable than fluoride, CFCs have even higher boiling temperatures. CFCs are effective solvents due to their polarity, and their boiling points make them acceptable as refrigerants. CFCs are less flammable than methane, in part because they have fewer C-H bonds, and in part, because the released halides, in the case of chlorides and bromides, quench the free radicals that keep fires going. The densities of CFCs are higher than their corresponding alkanes. In general, the density of these compounds correlates with the number of chlorides. CFCs and HCFCs are usually produced by halogen exchange starting from chlorinated methanes and ethanes. Illustrative is the synthesis of chlorodifluoromethane from chloroform: HCCl3 + 2 HF → HCF2Cl + 2 HCl Brominated derivatives are generated by free-radical reactions of hydrochlorofluorocarbons, replacing C-H bonds with C-Br bonds. The production of the anesthetic 2-bromo-2-chloro-1,1,1-trifluoroethane ("halothane") is illustrative: CF3CH2Cl + Br2 → CF3CHBrCl + HBr
Why is CFC harmful to humanity?
The earth's protective ozone layer is destroyed by chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), and halons, which shield the earth from damaging ultraviolet (UV-B) rays released by the sun. CFCs and HCFCs also warm the earth's lower atmosphere, causing global climate change.
Loss of stratospheric ozone can have serious consequences for human health and the environment. As a result of Ozone Depletion, Skin cancer and cataracts are becoming more common. The immune system of the human body reduces its efficiency. Moreover. Plantlife on land and in water is harmed by it and the production at ground-level ozone (smog) increases.
CFCs and HCFCs, in addition to destroying ozone, trap heat in the lower atmosphere, causing the earth to warm and temperature and weather to shift. HFCs absorb and trap infrared light or heat in the earth's lower atmosphere, and were originally developed to replace CFCs and HCFCs. HFCs, CFCs, and HFCs are all part of a wider group of greenhouse gases that contribute to global warming (GHGs). By the end of the century, greenhouse gases are predicted to have warmed the globe by 2.5 to 8 degrees Fahrenheit. Therefore, we witness a rise in the sea level, habitat loss and extinction of natural lives, an increase in the frequency of heavy rainfall and flooding, an increase of heat stress during summer and an increase in the risk of disease spread by insects and water.
Therefore, the Freon or the CFC is a harmful compound and may bring hazardous disasters. The challenge is to reduce it to a tolerable level. While reducing its need for technical action, producing it less is on us. In the next stage, we shall discuss the ways to reduce this enemy.
How to reduce CFC?
Refrigerants, particularly those used after the 1930s, are the most common source of CFCs. Coolant from outdated refrigerators, vehicles, air conditioners, and other machinery spills CFCs into the environment as liquids evaporate or find their way into the earth. Some countries' aviation laws still call for Halon, a CFC-containing coolant, to be used in fire suppression systems. For a long time, CFC-containing gases were employed in aerosol cans and propellant liquids. In 1999, they were taken out of the aerosol manufacturing process in favour of less toxic hydrocarbon substitutes. However, because CFC molecules have a lifetime in the stratosphere of 20 to 100 years, the damage done in prior decades is still being felt. People tend to forget about refrigerants and aerosol cans containing CFCs as they become older and more obsolete, allowing them to leak and further pollute the atmosphere. Researchers at the University of East Anglia are developing tools to identify local CFC exposure sources, such as obsolete CFC refrigerators. They gather air from the stratosphere and analyze it with mass spectrometers to see what chemicals are present in CFC pollution.
Therefore, to reduce the level of CFC emission we can take the following steps-
- Acknowledge the Ozone Layer Protection regulation. This Regulation prohibits the intentional release of controlled refrigerants into the atmosphere from motor vehicle air conditioners or refrigeration equipment containing more than 50 kg of refrigerant charge and encourages the use of approved recycling and recovery equipment to conserve the controlled refrigerants. Owners or operators of industrial/commercial refrigeration systems, as well as proprietors of garages, will be obliged to keep records on relevant repair services and the amount of CFC-based refrigerants consumed for enforcement and monitoring purposes. Vehicle scrap-yard owners must also keep records of the number of retired motor vehicle air conditioners and the amount of CFC-based refrigerants recovered from the decommissioned air conditioners.
- Not all refrigerator uses HCFCs as a refrigerant. You buy such a refrigerator.
- Such as refrigerators, may purchase aerosols that do not contain HCFCs or CFCs as propellants.
- When it comes to current air-conditioning and refrigeration equipment that uses HCFCs or CFCs, the refrigerant should be recovered or recycled whenever the equipment is overhauled. It's also worth thinking about replacing or adapting such equipment to run on non-HCFC refrigerants.
- When it's time to service your car's air conditioner, ensure sure the refrigerants are properly recovered and recycled rather than discharged to the atmosphere.
- Never dump your unused electronics outside.
By taking these simple measures, you also can stop CFC emissions. It’s not very hard you see. Come, let’s build a safer world together. Ultimately it is our planet.



